What Is the Hybridization of the Central Atom in Xef4
It has two lone pairs of nonbonding electrons on the central atom of Xenon. To summarize this blog post we can say that XeF4 has 36 valence electrons.
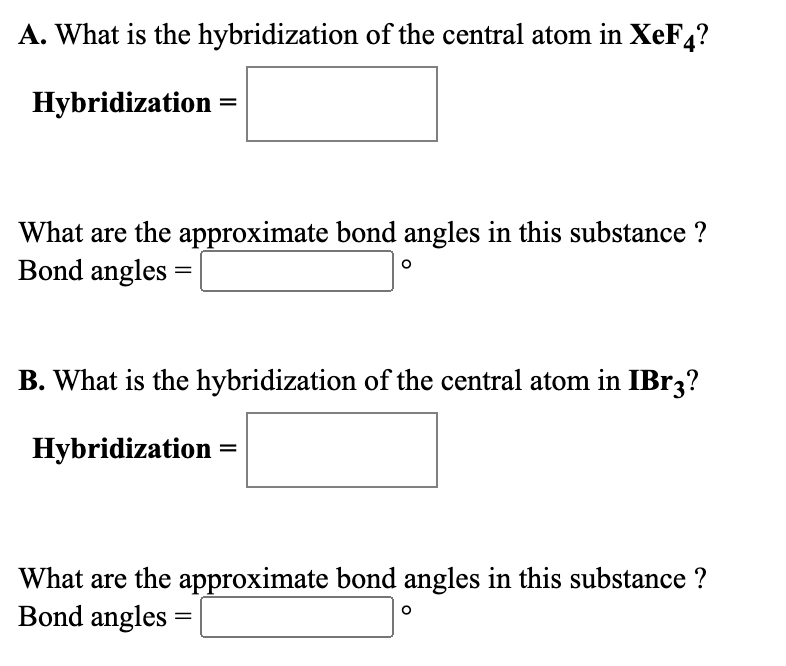
Solved A What Is The Hybridization Of The Central Atom In Chegg Com
XeF4 is a nonpolar molecule and has sp3d2 hybridization.
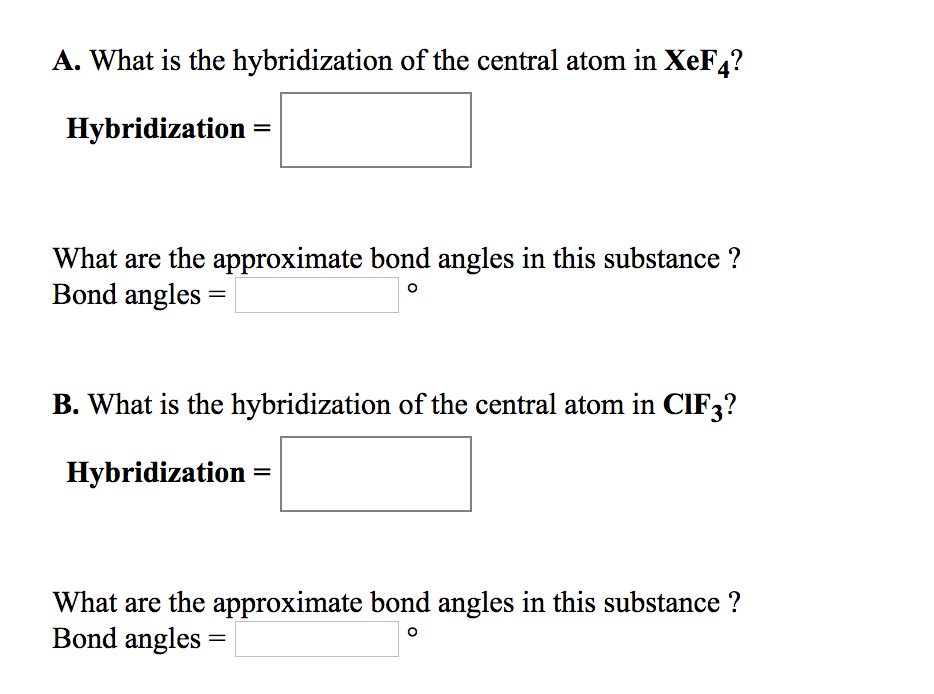
. Physical Chemistry Thermodynamics Structure and Change 10th ed Peter Atkins Julio de Paula 2014. Learn to determine the shape and hybridization of iodine in i3- with examples. This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry.
The molecule has octahedral electron geometry and square planar molecular geometry. The number of Ar atoms that we inhale with each breath is PV 102 1 105 Pa 0500 103 m3 23 N NA 6023 10 121 1020 1 1 RT 8314 J mol K 300 K The number of these that came from Caesars last breath is fN fN 153 1022 121 1020 185 102 The reciprocal of this result or 54 is the number of breaths needed to inhale one Ar atom. At the Geometry of Molecules we like knowing what.
Hyb of SCl2 can then be estimated using the formula below. In the SCl2 molecule sulfur is a core central atom with two Chlorine atoms connected to it. Lone pair on the central sulfur atom LPS Calculation for hybridization number for SCl2 molecule.
Hybridization of I3- is sp3d - Understand the molecular geometry and Hybridization of I3-. The number of SCl2 hybridizations No. It has two lone pair of electrons on sulfur.

Solved A What Is The Hybridization Of The Central Atom In Chegg Com

Hybridization Of Xef4 Xenon Tetrafluorid Youtube
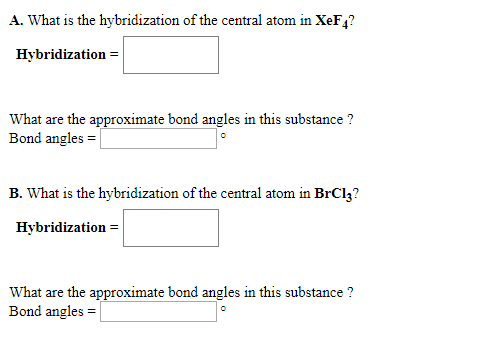
Solved A What Is The Hybridization Of The Central Atom In Chegg Com
Comments
Post a Comment